Simulating of Combustion of Natural Gas.
Simulating Combustion of Natural Gas
AIM:
1. Simulate the process of combustion on a model using a mixture of fuel and air.
2. Plot the variation of the mass fraction of different species in the simulation using line probes at different location of the model.
GIVEN PROBLEM:
The fuel species is methane and the fluid is air, which contains a mixture of oxygen and nitrogen
2. The velocity of air at the inlet is 0.5 m/s.
3.The velocity of fuel at the inlet is 80 m/s.
4. Part 1 : Combustion simulation to get mass fraction of different species like CO2,H2O,CH4,N2,O2,NOx and SOOT formation.
Adding water in fuel from 5% to 30% by mole and observing the results of NOxemission and SOOT formation.
NOTE:
- For Fuel, Use Methane-Air-2step as a mixture (It allows to add waterbin the fuel or air)
- For Soot formation, use one-step Soot Model.
THEORY:
1. COMBUSTION
Combustion is defined as achemical reaction in which a hydrocarbon reacts with an oxident to form products, accompanied by the release of energy in the form of heat.
Combustion manifests as awode domain during the design, analysis, and performance characteristics stage by being an integral part of various engineering applications like internal combustion engines,furnaces and power station conbustors.
2. Conbustion model for CFD Analysis
Comptational fluid dynamics modeling of combustion calls upon the proper selection and implementation of model suitable to faithfully represent the complex physical and chemical phenomenon associated with any combustion process.
The model should be competent enough to deliver information related to the species concentration, theri volumetric generation or destruction rate and changes in the parameters of the system like enthalpy, temperature and mixture density.
The model should be capable of solving the general transport equations for fluid flow and heat transfer as well as additional equations of combustion chemistry and chemical kinetics incorporated into that as per the simulating environment desired.
3. Possible types of Combustion Simulation possible in Ansys Fluent
The simulation of combustion can be divided into two types-
A. Combustion based on Mixing
- Non-Premixed Combustion
- In non-premixed combustion, fuel and oxidizer enter the reaction zone in distinct streams.
- It occours during direct injection or late injection of fuel in IC engines.
- Examples of non-premixedcombustion includes pulvarized coal furnaces, diesel internal-combustion engines and pool fires.
- Premixed Combustion
- In premixed combustion,fuel and oxidizer are mixed at the molecular level prior to ignition.
- Combustion occurs as flames front propagating into the unburnt reactants.
- Examples of premixed combustion include aspirated internal combustion engines, lean- premixed gas turbine combustors, and gas-leak explosions.
- Partially Premixed Combustion
- Partially premixed combustion systems are premixed flames with non-uniform fuel-oxidizer mixtures(equivalence ratios).
- Such falmes include premixed jets discharging into an inert atmosphere, lean premixed combustors with difussion pilot fames and cooling air jets, and imperfectly mixed inlets.
B. Combustion based on Phase
- Fluid Phase (Volumetric Reactions)
- Combustion takes place in the fluid region.
- Wall (Surface Reactions)
- Combustion takes place on the wall or a solid surface.
- Particles ( Surface Reactions)
- Combustion takes place on the Lagrangian particles.
- Combustion of coal is an example of particle combustion.
- Porous Region
- Combustion takes place in a porous region.
- The reactions in an after-treatment system is am example of combustion in the porous regions.
. Combustion Reactions
The general reaction of combustion is given as follows-
![]()
Balancing the given stoichiometric equation-
- a=1
- 2b=4 , b=2
- 2ar=2a+b,ar=2
- c=7.52ar , c=15.04
The above values corresponds to the number of moles of the given species.
AIR:2 moles of O2 +7.52 moles of N2
Mole fractions:
Mole fractions of O2=2/(2+7.52) =0.21
Mole fractions of N2 = 7.52/(2+7.52) =0.79
Mass fractions:
Mass fraction of O2=(0.21*16)/((0.21*16)+(0.79*14) ) = 0.23
Mass fraction of N2 = (0.79*14)/((0.21*16)+(0.79*14) ) = 0.77
Method:
Now load the geometry
Then ctrl+6 and set the pic like this

Then go to Design and select plane and click on the middle of x-y-z

Now we have to cut the geometry and get 1/4 th of the section.so to do this first select three object of the geometry by pressing ctrl and then go to prepare - split by plane and first select the first plane given in below pic then cut the lower section of the geometry then select the second plane and cut the front section of the geometry.



Then select the x-y plane of the three bodies and copy-paste these consequitively in another design.Before that in new design ctrl+6 and select sketch plane below to create a 2-d x-y plane in that design before pasting the planes.

Then go to Design - combine - select the overall geometry and click on the combine .

Then escape and now we can see the three surfaces has become a single surface.

Now cut the previous design keeping the new design close geometry and open mesh.Before closing geometry save the new design because in meshing new design may not be loaded so opening new tab import the new design geometry and then open meshing.
In meshing select element size 1mm , capture proximity and promixity gap factor 1 .

Now select names of the geometry.
Fuel inlet:

Air inlet:

Walls:

axis:

outlet:

Then update mesh and close it.
SET-UP:
After opening set up a warning message will appear due to axis boundary as we don't know whether it is symmetric or wall so we are changing the 2d space in physics-General to axisymmetric.
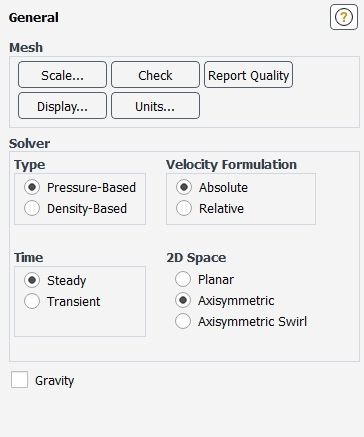
WE enabling the energy equation and viscous we are selecting like below pic

Species Mode:

Now we have to select the NOx and SOOT formation.



Boundary Conditions:
air inlet:


fuel inlet:


When adding water (say 80% ch4 and 20% H20)

Then in solution keep Method - SIMPLE
choose - Hybrid initialization
Number of iterations - 1200.
RESULTS:
In cfd post select location-pane. In plane select x-y plane , color Mode- variable, variable- temperature, mass fractions.
Now we have to draw charts to show the variation of temperature , mass fractions along the plane . To do it we have to draw lines to show the variations along the lines.with the help of the probe command we can track the geometry of the plane and draw lines accordingly.

point 1 point-2
line-1 (0.05,0,0) (0.05,0.08626,0)
line-2 (0.10,0,0) (0.10,0.08626,0)
line-3 (0.15,0,0) (0.15,0.08626,0)
Like this.
NOW go to the chart command

In chart - general select the title , in Data Series select new series and its location line , in x Axis select Y and in y Axis select Temperature/mass fractions.

Case-1:(CH4 100%)
RESIDUALS:

Temperature:


C02:
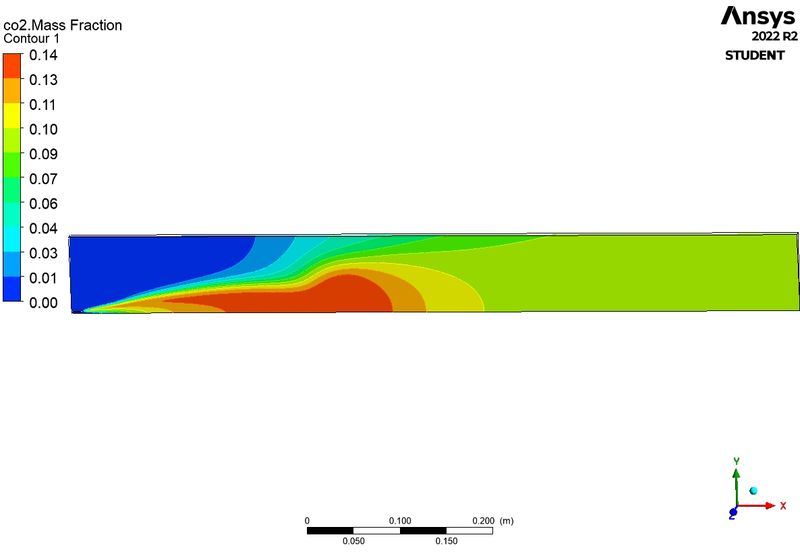

H2O:


CH4:


N2:

O2:


NOx:
NO:


N2O:


SOOT:


Case-2(95% CH4, 5% H2O)
RESIDUAL:

Temperature:


CO2:


H2O:


CH4:


N2:


O2:


NOx:
NO:


N2O:
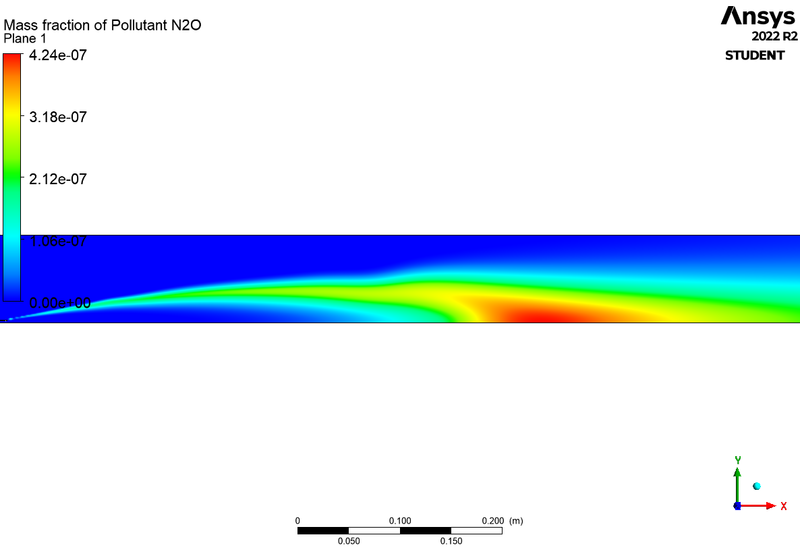

SOOT:


Case-3(80% CH4, 20% H2O):
RESIDUAL:

Temperature:


CO2:


H2O:


CH4:


N2:


O2:


NOx:
NO:


N2O:


SOOT:


Case-4(70% CH4, 30% H2O):
RESIDUAL:

Temperature:


CO2:


H2O:


CH4:

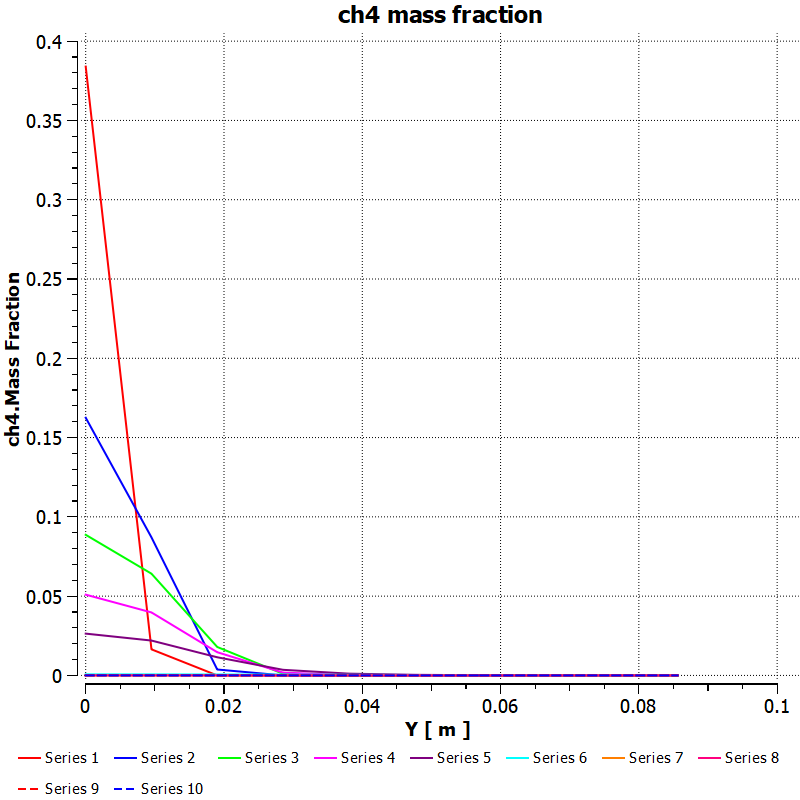
N2:


O2:


NOx:
NO:


N2O:


SOOT:


Data Table:
| CASE | % of Water | Temperature | Mass fraction OF N2O | Mass fraction OF NO | Mass fraction OF SOOT |
| 1 | 0 | 2281 | 3.59E-07 | 6.85E-03 | 5.50E-11 |
| 2 | 5 | 2330 | 4.24E-07 | 1.09E-02 | 9.84E-12 |
| 3 | 20 | 2270 | 3.30E-07 | 4.73E-03 | 2.96E-12 |
| 4 | 30 | 2195 | 2.815E-07 | 1.454E-03 | 1.101E-11 |
Conclusion:
- As per above observations, we can say that addition of water to the fuel helps in decreasing NOx and SOOT Formation.
- Water addition leads to increase OH radicals that might have a significant impact in SOOT Oxidation and reduce the SOOT formed in Gas Phase.
- Added water dilutes calories generatd by combustion. That decreases combustion temperature. If temperature reduces sufficiently, NOx cannot form in great concentration.
- We can see small decrease in temperature. Reducing combustion temperature means avoiding stoichiometric ratio. If this ratio is low, it generates lower concentration of thermal NOx.
- So, with these or various types of technologies, we can reduce NOx emissions and SOOT formation, which helps us to meet regulatory requirements by government. And we can reduce its harmful effects on environment and humans.
So briefly we can conclude that the simulation provides a complete temperature distribution along the geometry. Contours of various species can be studied.By adding water, the pollutant soot and NOx formation at the outlet is reduced. The plots of the same is also looked into.
REFERENCES:
1. https://www.sciencedirect.com/science/article/pii/S1876610217337724
2. https://link.springer.com/article/10.1007/s00773-015-0303-8
Comments
Post a Comment